Description
This zip file contains many different activities (68 pages of student handouts and 1 PowerPoint with a total of 54 slides) which can be used to compose a unit for chemistry students involving topics in bonding. Topics in this unit include properties of electronegativity and its relationship to bond type, covalent bonds (polar and nonpolar), molecular shape and symmetry, ionic bonding, Lewis dot structures, hydrogen bonding, relationship of bonding forces to melting and boiling points and more.
Two lab activities are contained within this package. One is an introduction to bonding lab requiring simple materials which can be purchased in most grocery stores or pharmacies plus some beakers or test tubes. The second lab uses an internet simulation to allow the student to construct molecular structures and examine bonding forces and simple molecular shapes.
The components of this lesson package will fit well with any high school chemistry course. Many of these materials may be useful for other physical science courses as well.
The components this unit addresses in the NGSS Standards and Common Core Standards are indicated at the end of this description.
Most documents are included in both word and pdf format to allow editing for specific teacher needs. The activities in this package are well suited for distance learning.
The specific contents of the learning package includes the following items (the page count for these items are actual student handouts as answer key page counts are not included):
-- Marzano self assessment scale including learning goals, NGSS and Common Core Reading/Writing Standards for students specific to this unit (2 pages)
-- Cloze notes handout for students with learning goals and Common Core Standards to accompany lesson PowerPoint (8 pages)
-- 57 slide PowerPoint to accompany the cloze notes
-- Bonding Reference Table (3 pages)
-- Bonding Worksheet # 1 (Electronegativity and Bond Energy) with answer key (3 pages/20 questions)
-- Introduction to Bonding Worksheet with answer key (1 page)
-- Bonding and Lewis Structures Worksheet with key (5 pages/25 questions)
-- Types of Chemical Bonds Worksheet with key (7 pages/47 questions)
-- Polarity and Chemical Bonding Worksheet with with answer key (8 pages/40 questions)
-- Drawing Covalent Bonds Worksheet with answer key (2 pages)
-- Bonding Reinforcement Activity with answer key (2 pages)
-- Introduction to Bonding Lab with Key (8 pages)
-- Models of Molecular Compounds: A Computer Simulation Chemistry Lab with key (8 pages)
-- Bonding quiz # 1 with key (1 page/9 questions)
-- Bonding quiz # 2 with key (4 pages/17 questions)
-- Bonding Exam with key (6 pages/40 questions)
Learning Goals
Upon the completion of this unit the student will be able to:
1. compare electronegativity values to determine the type of bond present in a compound.
2. represent the type of bond by drawing a model of ionic and covalent bond types.
3. state the properties associated with each bond type.
4. categorize all types of compounds into ionic, covalent or metallic bonds.
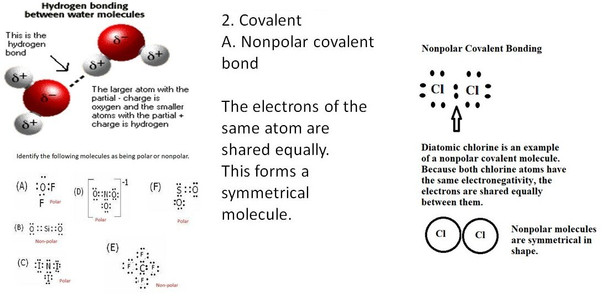
5. explain chemical bonding in terms of the behavior of electrons by demonstrating bonding concepts and using Lewis dot structures representing valence electrons.
6. determine the noble gas configuration an atom will achieve by bonding.
7. predict element combinations to form compounds with the use of drawings, animations and/or molecular models kits.
8. utilize Lewis dot structures in the determination of molecule polarity.
9. explain the properties of materials in terms of the arrangement and properties of the atoms that compose them by distinguishing among ionic, molecular, and metallic substances, given their properties.
10. compare the physical properties of substances based on chemical bonds and intermolecular forces.
NGSS Standard
Students who demonstrate understanding can:
HS-PS1-1. Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms.
Common Core State Standards Connections
ELA/Literacy
RST.9-10.7 Translate quantitative or technical information expressed in words in a text into visual form (e.g., a table or chart) and translate information expressed visually or mathematically (e.g., in an equation) into words.
SL.11-12.5 Make strategic use of digital media (e.g., textual, graphical, audio, visual, and interactive elements) in presentations to enhance understanding of findings, reasoning, and evidence and to add interest.
Terms of Use
Purchase of the product is for classroom use by the purchaser only. It is a violation for individuals, schools, and districts to redistribute, sell, or post this item on the Internet or to other individuals.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
View my complete High School Complete Chemistry Course