Description
This listing features two three-page tests about quantum chemistry featuring the electron. Version one is for the regular education students taking high school chemistry. Version two is a modified assessment for students with an IEP.
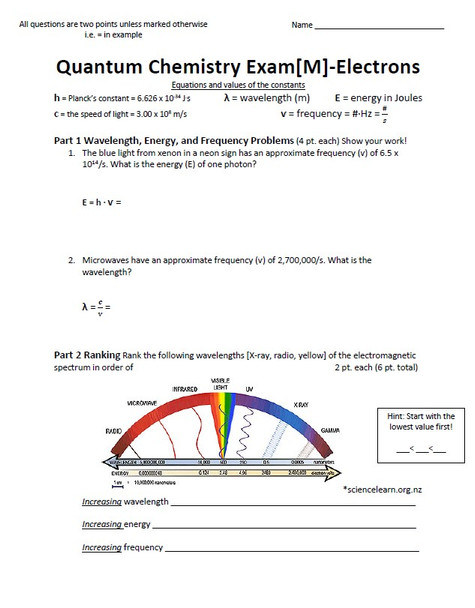
- In part one, students solve for the wavelength, frequency, and energy of a particle using the appropriate equation. I provided an equation bank for the two equations students will need on the regular test. I added a breakdown of what the variables mean on the header of page one for both test versions. I displayed the proper equation showing the isolated variable in the far left side of the work space on the modified test.
- In part two, students rank three different wavelengths of the electromagnetic spectrum by increasing wavelength, energy, and frequency.
- In part three, students write out an orbital diagram and answer a question.
- In part four, students write out three full electron configurations and two noble gas (abbreviated) electron configurations.
- In part five, students match the Aufbau Principle, Hund's Rule, and Pauli Exclusion Principle with their correct description
- Students answer four questions in section six.
- Students answer three true or false questions in part seven and correct the false statements to make them accurate.
- In part eight, students critique a blend of three orbital diagrams and electron configurations. If the shown layout or configuration is inaccurate, students state which rule appears violated before either describing or sketching the correct notation.
I provided a three-page key with the answers in bold, red font for each test.
This PDF assessment will become editable upon conversion to Microsoft Word using an Adobe Acrobat Reader DC app.
Hopefully, this user-friendly assessment will serve you well. I joined Amped Up! Learning as an author with seventeen years of publishing experience through several international science supply corporations.