Description
This zip file contains many different activities (37 pages of student handouts and a PowerPoint with a total of 42 slides) which can be used to compose a unit for Chemistry students on topics involving the Mathematics of Chemistry. Topics included in this unit are calculation of gram molecular mass, percent composition, water of hydration, the concept of the mole, mole conversions to gram molecular mass and numbers of molecules, mole relationships in equations and determination of empirical and molecular formulas. These lessons will fit well with the design of nearly any high school Chemistry class. It also may fit well with many physical science courses as well.
Many documents are included in both word docx as well as pdf format to allow editing for specific teacher needs.
The specific contents of the learning package includes the following items (the page count for these items are actual student handouts as answer key page counts are not included):
-- Marzano self assessment scale including learning goals and Common Core Reading/Writing and Mathematics Standards for students specific to this unit (2 pages)
-- Cloze notes handout for students with learning goals (8 pages)
-- 44 slide PowerPoint to accompany the cloze notes
-- Determining Water of Hydration with key (5 pages)
-- Mole Map (1 page)
-- Gram Formula Mass Worksheet with key (13 problems) (1 page)
-- Percent Composition with key (15 problems) (2 pages)
-- Percent Composition and Finding Empirical/Molecular Formula with key (2 pages)
-- Moles Conversions with key (20 problems) (4 pages)
-- Moles/Molecules and Grams/Moles/Molecules Worksheet with key (8 problems) (2 pages)
-- Mole-Mass Conversions Worksheet with key (10 problems) (2 pages)
-- Empirical and Molecular Formula worksheet with key (8 problems) (2 pages)
-- Mathematics of Chemistry Exam with key (25 mixed questions) (4 pages)
-- Water of Hydration Handout (1 page)
-- Mole Relationships in Equations with key (3 pages) (7 multi-step problems)
This unit has been correlated to the Common Core standards and the NGSS Standards. These standards are listed on the first page of the completion notes for the students and the Marzano self assessment.
Learning Goals
Upon the completion of this unit the student will be able to:
1. Determine the gram atomic mass of an element using the periodic table.
2. Determine the formula mass (gram formula mass, gram-molecular mass, molecular mass, molar mass) of a compound.
3. Determine the formula mass of a hydrated salt.
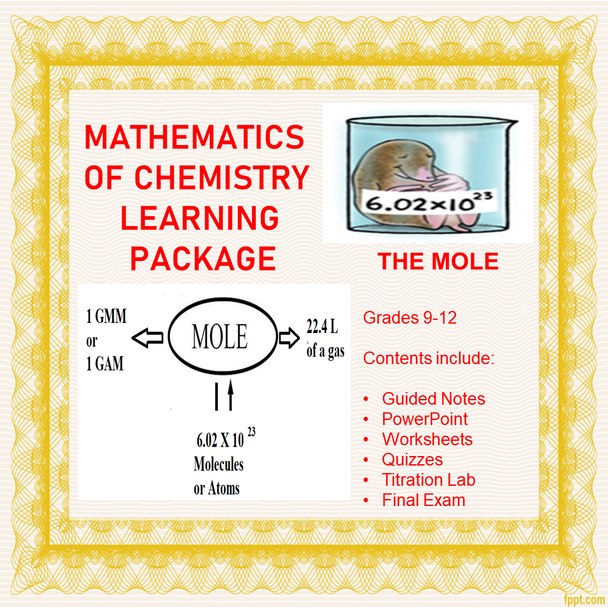
4. Know the definition of the mole.
5. Know Avogadro’s number and what it signifies.
6. Given the formula of a compound, determine the number of moles of each element (or water) present in one mole of the compound.
7. Given the formula of a substance, perform a variety of one-step mole conversions including but not limited to; moles to grams, grams to moles, moles to number of particles, and particles to number of moles.
8. Determine the percent composition of the elements in a compound, given the formula of the compound.
9. Determine the percent water in a hydrate, given the formula of the hydrate.
10. Perform 2 step mole conversions including; grams to number of particles and number of particles to grams.
11. Define the terms and distinguish between an empirical formula, a molecular formula, and a structural formula.
12. Given the empirical formula and the formula mass of a covalent compound, determine its molecular formula.
NGSS Standard
Upon the completion of this unit the student will be able to:
HS-PS1-7. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Common Core State Standards Connections:
Mathematics
MP.2 Reason abstractly and quantitatively.
HSN-Q.A.1 Use units as a way to understand problems and to guide the solution of
multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
HSN-Q.A.2 Define appropriate quantities for the purpose of descriptive modeling.
HSN-Q.A.3 Choose a level of accuracy appropriate to limitations on measurement when reporting quantities.
Terms of Use
Purchase of the product is for classroom use by the purchaser only. It is a violation for individuals, schools, and districts to redistribute, sell, or post this item on the Internet or to other individuals.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
View my complete High School Complete Chemistry Course