Atomic Structure Learning Activities (Distance Learning)
- Bulk Pricing:
- Buy in bulk and save
- Contributor:
- Monday's Rescue
- Grade Level:
- 9-12
- Product Type:
- Learning Package Bundle (Notes, PowerPoint, Lab, Homework)
- File Type:
- doc, pdf, ppt
- Pages:
- 44
- Answer Key:
- Yes
Description
This zip file contains many different activities (44 pages of student handouts and 2 PowerPoints with a total of 140 slides) which can be used to compose a unit for chemistry students involving the basics on Atomic Structure. Topics in this unit include characteristics of the basic subatomic particles, historical development of the model of the atom, isotopes and calculation of isotopic masses, interpretation of elemental symbols, electron configurations, emission spectra and more. While these lessons were originally designed to correlate to the performance indicators of the New York State Chemistry - Physical Setting curriculum, the components of this lesson may be easily used in other chemistry or physical science courses as well. The components this unit addresses in the NGSS Standards and Common Core Standards are indicated at the end of this description.
The components of this lesson package can easily be displayed to students using and LCD projector and may be readily modified into formats facilitating smartboard technology.
Many documents are included in both word docx as well as pdf format to allow editing for specific teacher needs.
The specific contents of the learning package includes the following items (the page count for these items are actual student handouts as answer key page counts are not included):
-- Marzano self assessment scale including learning goals and Common Core Reading/Writing and Mathematics Standards for students specific to this unit (2 pages)
-- Cloze notes handout for students with learning goals and Common Core Standards (9 pages)
-- 84 slide PowerPoint to accompany the cloze notes
-- Chemical Flame Tests Lab (4 pages)
-- Proton, Neutron and Electron Worksheet with key (1 page)
-- Atomic Structure Worksheet # 1 (Protons, Neutrons and Electrons in word and pdf with answer key (30 questions) (3 pages)
-- Atomic Structure Worksheet # 2 (electron configurations) in word and pdf with answer key at the end (7 pages/44 questions)
-- Atomic Structure Worksheet # 3 in word and pdf with answer key at the end (Isotopes and Models of the Atom) (6 pages/25 questions)
-- Atomic Structure Unit Exam in word and pdf format with key at the end(9 pages/50 questions)
-- 25 question Jeopardy review game in PowerPoint format (54 slides)
-- Ions and Isotopic Masses Worksheet in word and pdf with key (1 page)
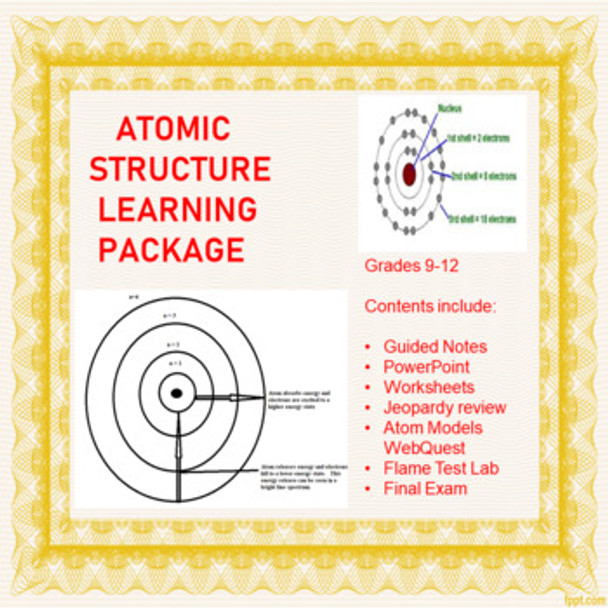
-- Atomic Structure Bohr model handout (1 page)
-- Models of the Atom Webquest with key (3 pages)
Learning Goals (Local)
Upon the completion of this unit the student will be able to:
1. identify the three subatomic particles in an atom and state basic properties of each particle.
2. tabulate the number of proton, neutrons and electrons in atoms, ions, or isotopes using the periodic table.
3. calculate the mass of an atom, the number of neutrons or the number of protons, given the two other values.
4. use the Bohr Model to describe the structure of an atom.
5. interpret and write isotopic notation.
6. determine the atomic mass from sample isotopic data.
7. identify an element by comparing its bright-line spectrum to given spectra
8. distinguish between ground state and excited state electron configurations.
9. explain the location of electrons using spdf sublevel notation.
10. discuss energy changes in the atom as electrons move between energy levels.
11. define and sketch valence electrons.
12. distinguish between ground state and excited state electron configurations.
13. explain the location of electrons using spdf sublevel notation.
14. discuss energy changes in the atom as electrons move between energy levels.
15. define and sketch valence electrons. Standards
NGSS Standards
HS-PS1 Matter and Its Interactions
Students who demonstrate understanding can:
HS-PS1-1. Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms.
HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
STAAR Standards
Readiness Standard
C.6.E express the arrangement of electrons in atoms through electron configurations and Lewis valence electron dot structures
Supporting Standard
C.6.A understand the experimental design and conclusions used in
the development of modern atomic theory, including Dalton’s Postulates, Thomson’s discovery of electron properties, Rutherford’s nuclear atom, and Bohr’s nuclear atom
Common Core State Standards
ELA/Literacy
RST.9-10.7 Translate quantitative or technical information expressed in words in a text into visual form (e.g., a table or chart) and translate information expressed visually or mathematically (e.g., in an equation) into words.
WHST.9-12.2 Write informative/explanatory texts, including the narration of historical events, scientific procedures/ experiments, or technical processes.
Mathematics
HSN-Q.A.1 Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
Terms of Use
Purchase of the product is for classroom use by the purchaser only. It is a violation for individuals, schools, and districts to redistribute, sell, or post this item on the Internet or to other individuals.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
View my complete High School Complete Chemistry Course