Isoelectronic Series and Analysis Worksheet Set for Chemistry
- Bulk Pricing:
- Buy in bulk and save
- Contributer:
- Parker's Products for the Sciences
- Lesson Category:
- Worksheet
- Grades:
- 10-12
- Answer Key:
- Yes
- Pages:
- 9
- Product File:
- Microsoft Word 2010
Description
Welcome! When chemistry students begin to learn about periodic trends, important concepts such as atomic instability and the desire for atoms to become isoelectronic with the nearest noble gas arise. Students seem to readily grasp what an ion is, but they often struggle to comprehend how ionization occurs or what is meant by an isoelectronic series.
The two activities in this listing will help chemistry students reinforce the understanding of and the relationship among key processes and terms such as ionization, cations, anions, parent atoms, and isoelectronic states. Mastery of the aforementioned concepts will help students better understand the rise of electrostatic forces of attraction responsible for ionic bonds in crystal lattices.
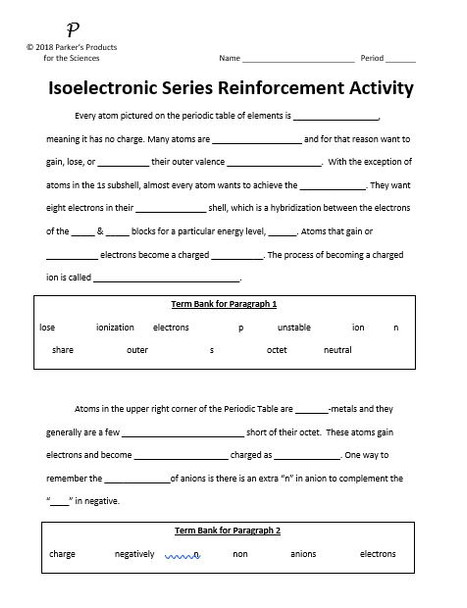
The first lesson is a full-page fill-in-the-blank activity. It contains four paragraphs, each with a word/term bank.
The second lesson is a full-page isoelectronic state identification and analysis sheet. Each section presents students with several rows of species in the form of atomic symbols. Some of the species are ions with the correct charge, while others are ions with an incorrect charge. Some of the species are parent atoms without a charge.
-
In the first section, students will circle the species (atomic symbol) in the row that are isoelectronic with the stated noble gas.
-
In the second section, students will circle the species in the row that are electron stable (isoelectronic with the nearest noble gas) and charge reactive (Ready to form an ionic bond)
-
In the third section, students will circle the species in the row that are electron unstable and not isoelectronic with its respective nearest noble gas.
-
In the fourth section, students will answer a series of short answer questions about ionization and isoelectronic series.
This PDF file will become editable upon conversion to a Microsoft Word Document using an Adobe Acrobat Reader DC Program. It includes a two full-page keys with the answers in red font.
I offer dozens of quality field-tested, innovative, practical, and user-friendly products for different fields of science - biology, chemistry, field ecology, physical science, earth science, space science and human anatomy and physiology.
Thank you for your interest. Check out my store, Parker's Product for the Sciences, for other engaging science activities.